The ionizing properties of ultraviolet radiation have already been the target of researchers and designers who take advantage of it in different ways, or even give warning about its presence.
In excess, when it affects our skin it can cause genetic mutations in cells that leads to uncontrolled reproduction. In the environment, it can cause insects disorientation affecting the ecological balance. In domestic and laboratory equipment it can be used in photo oxidation and sterilization processes
Ultraviolet radiation
To understand how we can take advantage of ultraviolet radiation in practical applications, we need to know its nature, that is, to know exactly what it is. We have some additional articles on the site that the reader can consult, but we will take a different approach here just with the aim to better understand its biological applications.
Ultraviolet radiation or ultraviolet light is a form of electromagnetic radiation, that is, just like light, it consists of electromagnetic waves with a certain frequency and wavelength. Thus, we can locate it precisely in the spectrum of electromagnetic waves that includes visible light and infrared radiation, as shown in figure 1.

Note that below the visible range, infrared radiation has lower frequencies and above the violet in the visible range, we have ultraviolet radiation, with higher frequencies and shorter wavelengths.
Electromagnetic radiation can be described as small “energy packets”, in the case of light called photons, which carry a fixed amount of energy. These packets are called “quantum” (plural quanta – from Latin) and their energy depends on their frequency.
The higher the frequency and therefore the shorter the wavelength, the greater the amount of energy these particles carry.
This means that ultraviolet radiation photons carry much more energy than infrared light. In fact, we divided the ultraviolet spectrum into 3 bands, with different energies bands and, therefore, different penetrations.
- Ultraviolet A - UVA
- Ultraviolet B - UVB
- Ultraviolet C - UVC
The one with the greatest energy potential and, therefore, the greatest penetration is the UVC.
But what does this mean for living (and non-living) organisms?
Ionizing Potential
When an ultraviolet radiation photon hits an atom, it can cause the atom to absorb it with the jump of an electron to a higher level of energy, as shown in figure 2.
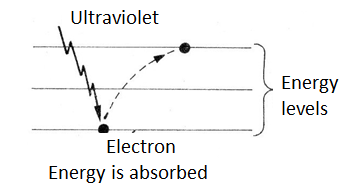
Eventually, the atom can return this energy by emitting a new photon. For example, if the electron returns to a lower level, but not the original, in a first jump, the energy emitted may fall in a lower frequency range, as shown in figure 3.
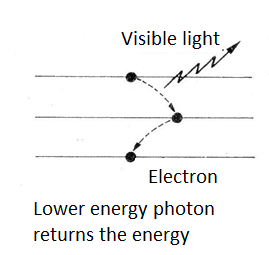
However, it can happen that the photon energy is enough to pull the electron out thus ionizing the atom. If the atom is part of a larger molecule, for example, an organic molecule, this can cause the molecule to break causing its destruction.
This is what happens when the highest energy ultraviolet radiation (UVC) reaches a polymer. This type of material has molecules formed by long atoms chains. If they are broken, the material loses its properties. It can be rough, brittle, change color, etc.
In the case of a virus, for example, ultraviolet radiation can disrupt the proteins molecules that form its external cover and with that it dies.
In other types of microorganisms, the same occurs. The radiation breaks up vital molecules, causing their death.
Ultraviolet sources
A natural source of ultraviolet radiation is the sun. For this reason, sunbathing is not recommended during periods when it is most intense. However, we need solar radiation so that it helps in the vitamin D synthesis. Acting on the substances in our body, with its ionizing potential it helps in the reactions that form it.
Artificially, a way to obtain ultraviolet radiation is through special lamps, such as ultraviolet fluorescents, in circuits as shown in figure 4.
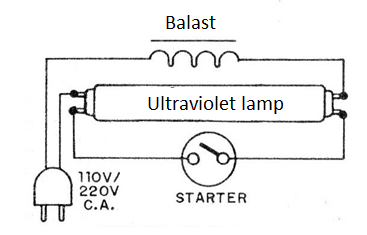
See that it is not a simple “black light” lamp that emits the less penetrating ultraviolet A (UVA), but rather a type of lamp used in old EPROM memory erasers (UVC), much more penetrating.
In article in our site in Portuguese, for example, we describe a power control for a photo-oxidation device used at the Oceanographic Institute of the University of São Paulo, which we helped to develop in 1998. (figure 5)

This device produces radiation in the 250 nm (UVC) range.
The third source, which is now available for the most diverse projects, thanks to the development of semiconductor technologies, is the one that makes use of LEDs.
Ultraviolet LEDs
Current LED technology allows devices to be developed relying on the ultraviolet radiation emission band with ease.
We can range from small, portable, battery-powered applications like sterilizers for surgical tools or battery-powered food, to larger ones. In the Corona Virus epidemic, for example, researchers from USP (Sao Paulo University – Brasil) developed an ultraviolet squeegee that, in addition to exercising its basic function of cleaning, also sterilizes by applying ultraviolet radiation. (figure 6)

For application developers using ultraviolet LEDs, it is necessary to take into account the type of radiation to be used (UVA, UVB or UVC), the intensity and possible side effects. This radiation should not affect people's eyes.
Thus, not only the technical development must be considered, but also the scientific basis that requires the support of proper documentation or specialized personnel.
LEDs in the market
Numerous ultraviolet LED manufacturers make their components available through Mouser Electronics. Manufacturers such as Vishay, Wurth, OSRAM, Lumileds, Luminus Devices provide LEDs covering the three ultraviolet bands.
Next, some UV LEDs examples that can be used in new projects.
Note: Enter UV LED in Mouser's search to access a large number of types from all manufacturers.
VLMU35CM-280 -120 - Vishay
Datasheet at: https://br.mouser.com/datasheet/2/427/vlmu35cmxx-280-120-1767424.pdf
These ultraviolet LED for wavelengths from 265 to 285 nm (UVC) from Vishay, available from Mouser, have their application indicated for medical areas such as germ sensing, DNA, etc.

The emission power is 15-mW under a 100-mA current.
WL-SUMW - High power ultraviolet LED - Wurth
More information: https://br.mouser.com/new/wurth-elektronik/wurth-high-power-uv-led/
These LED emit ultraviolet radiation in the A range, at wavelengths of 385 nm, 395 nm and 405 nm. The luminous flux is between 850 mW and 1.1 W depending on the type.



